研究發(fā)現(xiàn)植物草酸代謝途徑關鍵酶影響玉米營養(yǎng)品質
 ??????
??????
?  ? ?
? ?
9月10日,The Plant Cell?在線發(fā)表了中國科學院分子植物科學卓越創(chuàng)新中心/植物生理生態(tài)研究所巫永睿研究組題為Maize Oxalyl-CoA Decarboxylase1 Degrades Oxalate and Affects the Seed Metabolome and Nutritional Quality?的研究論文����。該研究克隆和功能解析了玉米草酸降解途徑中的關鍵酶——草酰輔酶A脫羧酶,揭示了草酸代謝參與籽粒儲藏物質積累和營養(yǎng)品質形成的分子機理����。
草酸是最簡單的二元酸�����,在植物體內的含量非常高��。草酸在調控金屬脅迫��、離子平衡和昆蟲防御等方面起積極作用���。然而,過量草酸不僅會影響植物自身發(fā)育�,也會影響包括鈣元素在內的多種礦物金屬礦物元素的利用;人體從食物中攝入草酸過多會和鈣形成草酸鈣,誘發(fā)形成腎結石���。有報道顯示��,植物體內存在草酸合成和降解途徑��,其中一條降解途徑由四種酶共同作用�,分別為草酰輔酶A合成酶�����、草酰輔酶A脫羧酶��、甲酰輔酶A水解酶和甲酸脫氫酶��。草酰輔酶A合成酶可以催化草酸形成草酰輔酶A��,接著草酰輔酶A在脫羧酶的作用下形成甲酰輔酶A���。草酰輔酶A合成酶在多種植物中均被發(fā)現(xiàn)��,然而卻未有草酰輔酶A脫羧酶的報道���,在農作物玉米中���,草酸的降解代謝途徑還未知,草酸與玉米籽粒發(fā)育��、營養(yǎng)物質存儲和品質調控的關系也不清楚��。
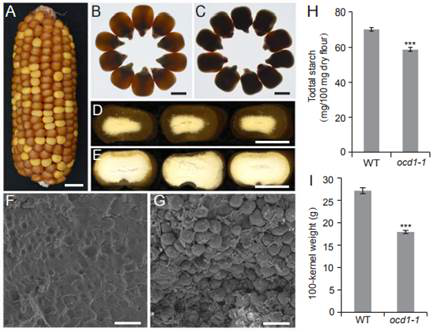
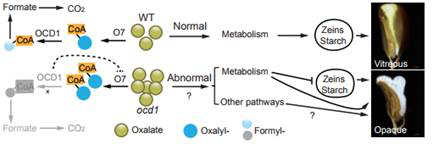
在此項研究中����,巫永睿研究組克隆了玉米草酰輔酶A脫羧酶(Oxalyl-CoA Decarboxylase1,OCD1)基因�,該基因突變以后籽粒胚乳呈現(xiàn)出粉質的表型,同時籽粒的儲存物質合成和粒重也發(fā)生下降��。由于沒有商業(yè)化的草酰輔酶A脫羧酶底物草酰輔酶A�����,研究人員嘗試了多種方法�,合成了較高純度的草酰輔酶A�。體外和體內的酶活實驗證實草酰輔酶A脫羧酶可以降解草酰輔酶A產生甲酰輔酶A和二氧化碳。同時���,研究人員還發(fā)現(xiàn)早先克隆的玉米經(jīng)典高賴氨酸突變體基因opaque7(o7)編碼草酰輔酶A合成酶��,并證明O7可以催化草酸形成草酰輔酶A����。另外,靶向和非靶向代謝組學分析發(fā)現(xiàn)��,玉米草酰輔酶A基因突變后籽粒胚乳的能量代謝�����、糖類��、氨基酸以及激素含量均受到顯著影響�����。該項研究闡明了玉米草酸代謝的前兩步反應��,并揭示了草酸降解途徑與籽粒胚乳發(fā)育����、代謝和營養(yǎng)品質的關系,為將來遺傳改良草酸含量較高的蔬菜(如菠菜)等提供了候選基因和分子機制��。
該工作主要由巫永睿研究組副研究員楊俊和博士生付苗苗合作完成���。博士生冀晨����、黃永財參與了相關工作。研究工作得到科技部��、國家自然科學基金�����、中科院等的資助���。(來源:上海生命科學研究院)
?
Maize Oxalyl-CoA Decarboxylase1 Degrades Oxalate and Affects the Seed Metabolome and Nutritional Quality
?
Abstract??The organic acid oxalate occurs in microbes, animals and plants; however, excessive oxalate accumulation in vivo is toxic to cell growth and decreases the nutritional quality of certain vegetables. However, the enzymes and functions required for oxalate degradation in plants remain largely unknown. Here, we report the cloning of a maize (Zea mays) opaque endosperm mutant that encodes oxalyl-CoA decarboxylase1 (EC4.1.1.8; OCD1). Ocd1 is generally expressed and is specifically induced by oxalate. The ocd1 mutant seeds contain a significantly higher level of oxalate than the wild type, indicating that the ocd1 mutants have a defect in oxalate catabolism. The maize classic mutant opaque7 (o7) was initially cloned for its high lysine trait, although the gene function was not understood until its homologue in Arabidopsis thaliana was found to encode an oxalyl-CoA synthetase (EC 6.2.1.8), which ligates oxalate and CoA to form oxalyl-CoA. Our enzymatic analysis showed that ZmOCD1 catalyzes oxalyl-CoA, the product of O7, into formyl-CoA and CO2 for degradation. Mutations in ocd1 caused dramatic alterations in the metabolome in the endosperm. Our findings demonstrate that ZmOCD1 acts downstream of O7 in oxalate degradation and affects endosperm development, the metabolome, and nutritional quality in maize seeds.